RESEARCH BRIEFING – WEEK IN REVIEW
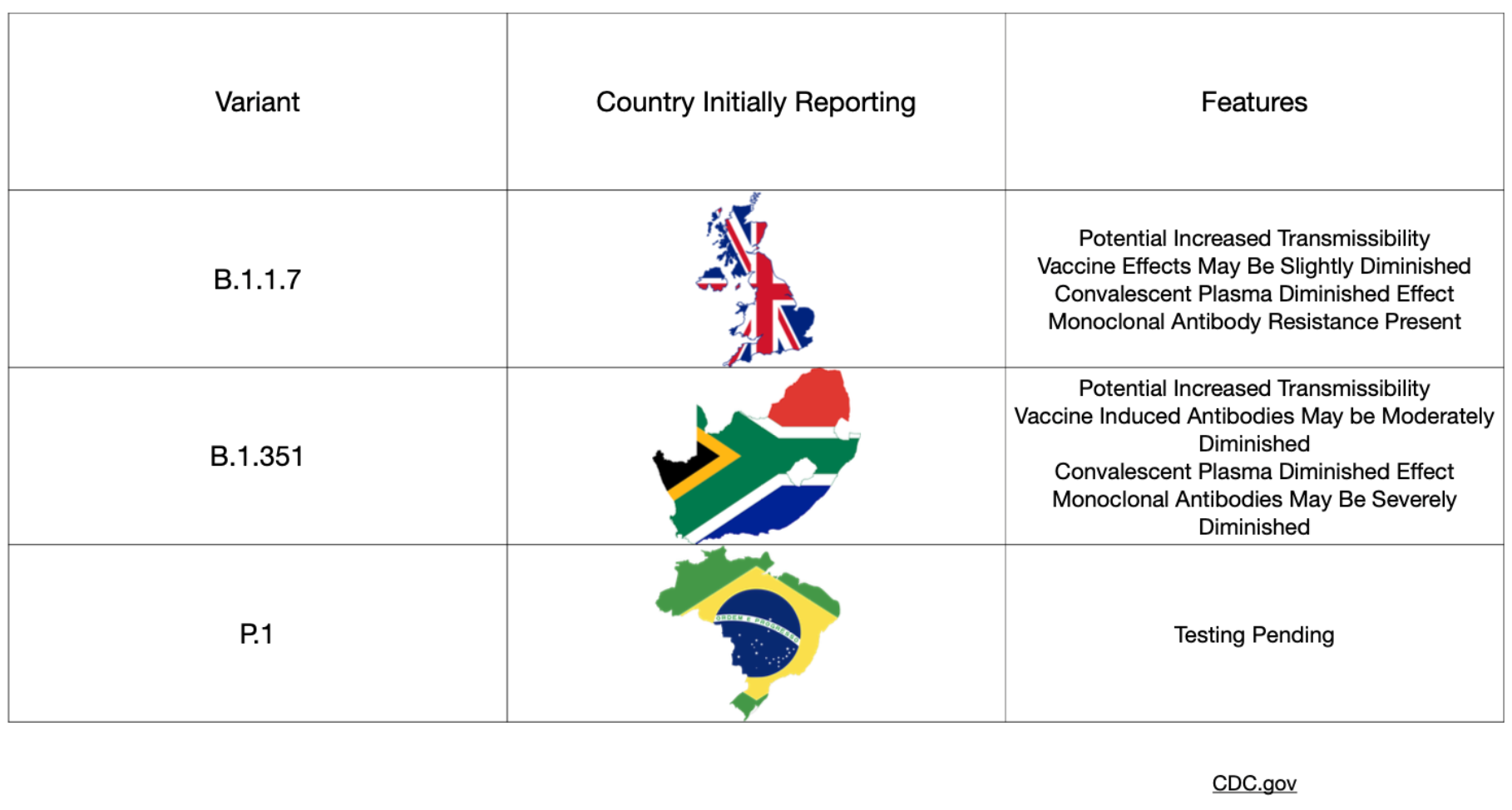
The covid-19 variants have dominated the news lately with significant focus paid to the UK (B.1.1.7), Brazilian (P.1), and South African (B.1.351) mutations. Concerns have been raised about increased infectiousness and the potential for increased mortality associated with these variant strains. In particular, many worry that the South African strain has even higher rates of transmission and imposes a higher viral burden than the UK version. Along with these concerns, questions have arisen regarding the effectiveness of our current vaccines and of natural immunity from previous infection with SARS-CoV-2. A recent group of preprints sought to address these questions.
The first preprint from researchers in the United States investigated the effectiveness of the mRNA-1273 vaccine (Moderna) against the aforementioned global variants. The Moderna vaccine previously demonstrated ~94 percent efficacy in Phase 3 data published last month and reviewed here at Brief19. A key feature of these variants is mutation of the spike proteins (the proteins studding the outer shell of the SARS-CoV-2 virus) with more extensive changes seen in the UK variant. Changes to parts of the protein called the "receptor binding domain" and "N-terminal domain" potentially have the ability to affect the binding of monoclonal antibody treatments, and crucially, possibly vaccine-induced antibodies too. This paper presents results from non-human primates and human subjects who received the Moderna vaccine. Blood samples from subjects were tested against multiple strains of the virus including the UK and South African mutations. Importantly and somewhat reassuringly the vaccine in these tests shows activity against the mutations—though some reduced effects were seen when exposed to the South African mutation in particular.
A second preprint investigated whether antibodies generated after a natural course of infection would provide resistance to the South African variant. In this very small study, convalescent plasma collected from six adult patients who were hospitalized with covid-19 in South Africa were then tested against the mutated strain. These results found that the natural antibodies demonstrated significant variation between the six test subjects, which raises concern that previous infection may not provide enough protection from variant exposure in all cases. These findings also imply that vaccines targeting the spike protein could only mount a weak response. The clinical implications of this are not understood.
A third preprint also addressed this increased resistance of the UK and South African variants to antibody neutralization. This study queried blood samples from those who had already received vaccination and those with naturally occurring antibodies generated after an infection (convalescent plasma), as well as efficacy of monoclonal (lab manufactured) antibodies. A total of 12 monoclonal antibodies were assessed along with convalescent plasma from 20 patients, plus samples from 12 people who enrolled in the Moderna Phase 1 study. Similar to the aforementioned preprints, the findings were concerning—the UK and South African variants demonstrated resistance to monoclonal antibodies, reduced efficacy of convalescent plasma, and a loss in vaccine activity ranging from 2 to 8.6 times less effective in the UK and South African variants, respectively. Again, the degree to which this would have a clinical impact is not known.
Moderna released a statement on January 25 hoping to assuage such fears, stating that their vaccine showed robust activity against these new strains. The company acknowledged the reduced effect, but still believe that titer levels generated by the vaccine should be protective. Moderna also suggest that the waning efficacy could be bolstered by a booster vaccine in the future.
In summary, these findings raise concern about potential for new infections, even in those previously infected or vaccinated. The usual scrutiny must be applied to these studies given they are preprints and all contain very small subject numbers. We must not let our guard down and continue safe practices of mask wearing and social distancing. Given the rapid appearance of mutations, the likelihood of annual vaccinations could be inevitable. The single best way to limit the emergence of even more worrying mutations remains to limit the spread of SARS-CoV-2. The fewer replication cycles it undergoes, the fewer mutations will occur. 29 January 2021.
Among the many possible treatments for covid-19, therapeutic anticoagulation—that is, treating patients with high dose blood thinners as though they had developed abnormal blood clots—has been of particular interest. Much of this has been driven by our knowledge of the covid-19 disease process, which appears to include a propensity towards potentially dangerous blood clot formation. Despite popular support for this approach among many healthcare providers on the frontlines of the covid-19 pandemic, the evidence supporting this approach has been, to date, largely gleaned from retrospective and observational studies. Such studies are prone to significant bias because whether or not patients received a treatment in such studies is not based on randomization but, rather, the subjective judgement of a treating clinician.
Several large randomized trials are underway to answer whether or not patients with covid-19 should receive anticoagulation. In late December 2020, three large randomized trials of full-dose anticoagulation for patients who were critically ill with covid-19 were halted due to futility and the potential that harm was being caused by the blood thinning medications.
Last week, the National Institutes of Health reported on another subgroup of test subjects, this time a group of more than 1,000 patients sick enough to be hospitalized but not ill enough to require either admission to an intensive care unit or invasive mechanical ventilation (i.e. intubation). During their hospitalization, patients were randomized to either therapeutic ('full-dose') anticoagulation (the drugs used included enoxaparin, heparin, dalteparin, and tinzaparin) or prophylactic dose anticoagulation. The researchers now report a 99 percent probability that therapeutic anticoagulation was superior to prophylactic anticoagulation in this patient population in preventing patients from needing mechanical ventilation or other forms of organ support. However, we do not yet know if there was an eventual difference in mortality.
These data suggest that there appears to be a sweet spot for anticoagulation in covid-19. In order for patients to benefit, they can neither be too sick nor too well. This may be because critically ill patients are more susceptible to the side effects of anticoagulation (such as clinically important internal bleeding), or perhaps they are already too sick for the intervention to make any real difference. The full trial results are not yet available and so it remains possible that the complete data will tell a different story. Nevertheless, it is likely that patients admitted to the hospital with non-severe covid-19 may soon be receiving full-dose anticoagulation routinely. 27 January 2021.