RESEARCH BRIEFING
Pediatric coronavirus vaccines trials won't report on efficacy. Why that's not a problem and how to sign children up
Results from adult coronavirus vaccine trials have made some of the only uplifting headlines of the covid-19 pandemic. With three options currently available in the United States, all of which appear to provide 100 percent protection against hospitalization and death, a light at the end of the tunnel is finally real.
However, as the vaccine rollout reaches more and more of the adult population, our attention will soon turn to kids. As one of us wrote in the New York Times yesterday, pediatric vaccinations will soon be a key in ending the pandemic. That's because unless we vaccinate enough children, there's a real possibility that a variant that evades the current vaccines or causes worse disease in children will emerge. While it's true that so far kids have mainly done well with natural infection, they are still an important "breeding ground" for mutations in the SARS-CoV-2 genetic code, which could ruin much of our progress. In addition, parents may be less enthusiastic to rush their kids to vaccination, thinking that the disease poses little threat to their children, but that the vaccine might have unknown side effects. However, even though the rate of serious covid-19 in children is low, the rate of serious allergy to the vaccine (or any other significant event) remains far lower, especially when under medical supervision.
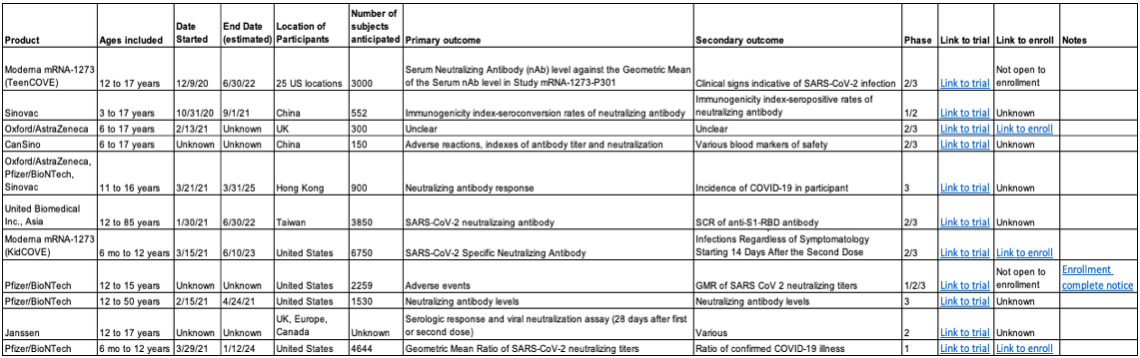
The problem is that of the eleven pediatric coronavirus vaccines that we know about (see table), none of them will report on how well the vaccine prevents non-adults from developing serious covid-19 or hospitalization. That's by design. Currently, the virus causes serious disease in kids too infrequently for a trial to detect a difference between the vaccine and placebo, unless it were to enroll hundreds of thousands of kids. But as stated, the goal is not just to protect kids from the disease, but all of us from having the vaccines in use become obsolete because of new variants that might emerge from pediatric infections.
As the table we have put together shows, the trials will primarily be reporting on safety and immune responses measured from blood taken days-to-weeks after the vaccinations. That means that doctors and other clinicians won't be able to tell parents that the vaccine has been proven to prevent serious pediatric disease (even though it likely will do exactly that). So, we have to rally the troops of our nation's parents to vaccinate their kids, both to protect against rare pediatric cases (and the downstream inflammatory syndromes), but also for "the rest of us." Right now, the more adults get vaccinated, the fewer kids will be exposed to the virus. But once enough adults have been inoculated, we'll be looking to our kids to protect us all from a variant that could prolong, or seriously worsen, the covid-19 pandemic.
POLICY BRIEFING
Last week, we reported on new data showing that very few vaccinated healthcare workers subsequently became infected with SARS-CoV-2. However, another study showed that at least one of the variants of concern currently being tracked did little to prevent mild or moderate cases of covid-19. Regardless, it's possible that any subset of vaccinated individuals who does become infected with the coronavirus may or may not be able to transmit the virus. It remains quite possible that even though such individuals did not have sufficient immunity to prevent their own infection, they may still have enough vaccine-induced antibodies to eliminate their ability to spread the virus, or maybe to limit it to a shorter window or shorter distances.
You might think that the vaccine trials looked at this. You would be incorrect. Achieving this would not have been terribly difficult. Investigators simply would have had to follow the household contacts of trial volunteers, checking for rates of infection among household members of those who had been vaccinated and comparing the findings to those in the placebo arm of the trial. Alas, that never happened.
Enter the National Institute for Allergy and Infectious Diseases (NIAID), directed by Dr. Anthony Fauci. The NIAID just announced a study that will be carried out at twenty-two United States universities. The study is called PreventCovidU. The website is explicitly (if awkwardly) geared towards Generation Z students, and includes abbreviations that "the kids use," like "TL;DR" ("too long, didn't read"), and "jk" (just kidding) in order to try to break the ice.
Unlike other studies of the vaccines in the US so far, participants will be required to supply daily swabs. In addition, over 25,000 "close contacts" will be invited to take part in the trial, so that researchers can see how often infected participants spread the virus, and to how many of their contacts. Of course, asking students to remember who they crossed paths with (or partied with) in the last few weeks, could be a challenge, possibly rendering the eventual data that is gathered somewhat difficult to fully interpret.